Connect With a Community of Viral Vector Innovators in 2027
With cost, consistency, and compliance continuing to challenge viral vector manufacturing, biopharma teams are actively seeking partners who can help them scale safely and efficiently. The 4th Viral Vector Process Development & Manufacturing Summit offered a focused opportunity to position your brand as a trusted solution provider at the center of these discussions.
As the only industry-led meeting dedicated to overcoming CMC and process development barriers for AAV, LV, and emerging vector systems, this annual summit connects you directly with the decision-makers driving change. Through technical case studies, analytical deep-dives, and over six hours of structured networking, you’ll engage with the experts tackling challenges around yield optimization, analytical consistency, and scalability - making this the ideal forum to position your solutions, strengthen partnerships, and connect with the leaders transforming viral vector manufacturing.

Why Partner With Us?
Tailored Networking Access
Meet the viral vector developers, CMC strategists, and MSAT leaders driving upstream and downstream innovation across AAV, LV, and emerging vector systems. Connect directly with the technical decision-makers seeking CDMO expertise, advanced bioreactors, analytical platforms, and automation technologies to strengthen process performance.
Stay Ahead of Manufacturing Innovation
Gain insider access to the latest case studies on yield improvement, process intensification, analytical standardization, and regulatory harmonization. Stay at the forefront of trends shaping vendor partnerships and technology adoption across the global viral vector landscape.
Strategic Brand Visibility
Position your company as a key enabler of scalable and compliant viral vector manufacturing. Through pre-event marketing, on-site branding, and speaking opportunities, your solutions, from plasmid supply to purification systems,will be showcased to a focused audience of biopharma and regulatory stakeholders.
Lead the Conversation
Deliver a thought leadership presentation or join an expert panel to demonstrate how your systems, assays, or CDMO services are reducing COGs, improving consistency, and enabling next-generation GMP manufacturing for advanced therapies.
Key Services & Solutions
Our attendees from biopharma are looking for service and solution providers with capabilities in the below areas but not limited to:
1
CDMO & End-to-End Manufacturing Services
Providing scalable GMP manufacturing, process development, and tech transfer capabilities to support vector production from preclinical through commercial stages.
2
Bioreactor & Upstream Processing Technologies
Supplying advanced bioreactor systems, transfection platforms, and suspension culture solutions to boost titres, reduce plasmid dependency, and improve scalability.
3
Downstream Purification & Filtration Solutions
Delivering chromatography, membrane, and continuous purification systems to enhance recovery, consistency, and process throughput.
4
Analytical & Characterization Services
Offering high-resolution analytical tools, bioassays, and release testing solutions to strengthen comparability, potency, and regulatory confidence.
5
Automation, Robotics & Digital Manufacturing
Enabling process control, AI-driven analytics, and closed-system manufacturing to improve reproducibility and reduce human error.
6
Raw Materials, Plasmids & Critical Reagents
Providing GMP-grade plasmids, cell lines, and raw materials to secure supply chains and minimize variability across batches.
7
Regulatory & CMC Consultancy
Guiding developers through evolving global expectations, pharmacopeial alignment, and platform designation pathways to accelerate approvals.
8
Quality Control & Process Analytical Technologies (PAT)
Supplying integrated QC tools and real-time monitoring solutions to ensure batch consistency, impurity clearance, and process validation under GMP.
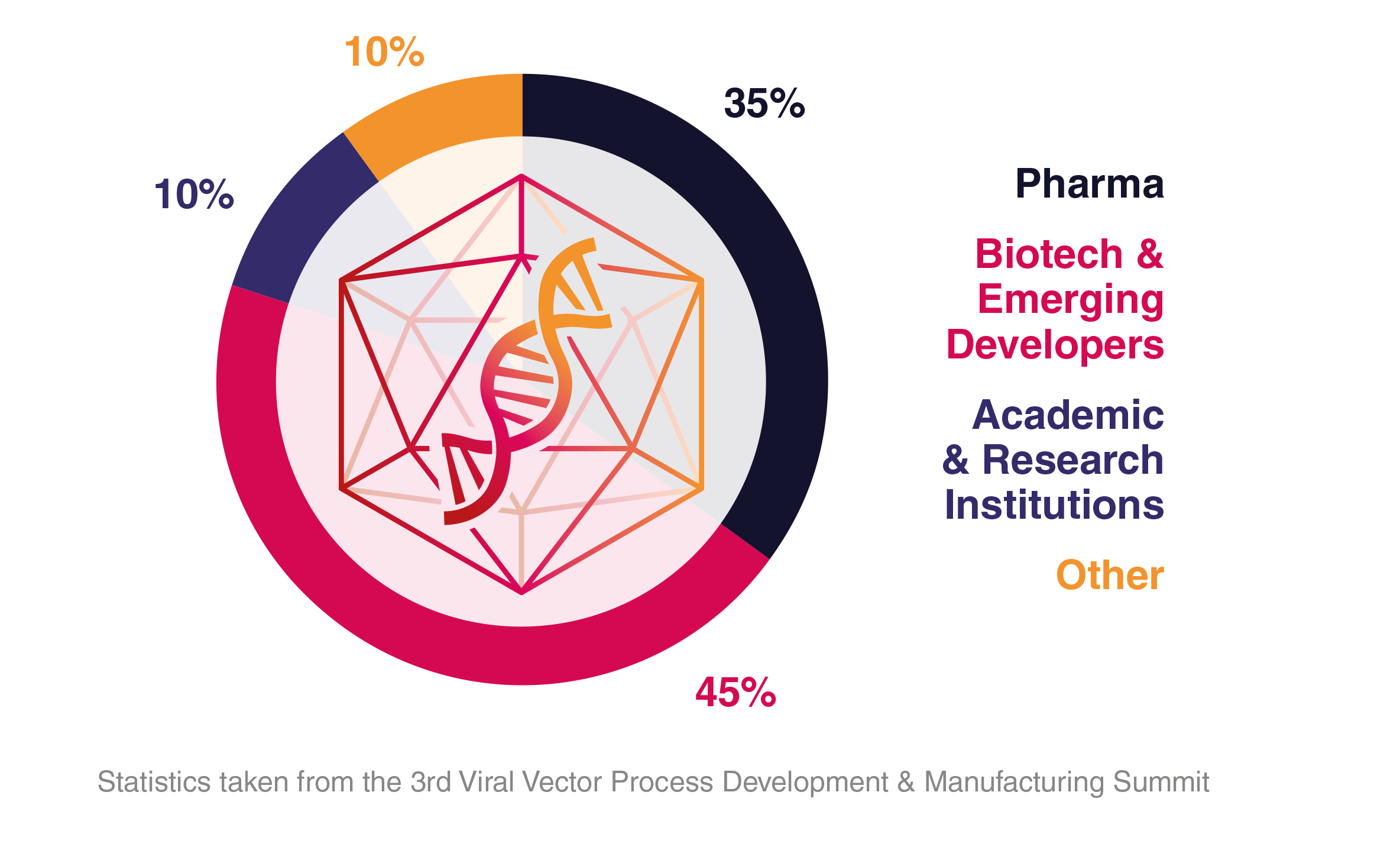
Audience Composition
Company Type
Attendee Seniority

Attending Companies Included









Get in Touch
Take advantage of our bespoke sponsorship opportunities to achieve your commercial goals. Email us if you would like to get involved and discuss a bespoke package suited to your needs.



